Recently there has been quite a few recalls and updates in the CPAP industry, causing many to wonder if their particular CPAP device, mask, accessories and cleaning supplies are still safe. Our focus has been and continues to be on our patients’ safety, wellness, and comfort, with the mission of a good nights’ sleep. In aligned with our mission, we strive to keep our patients and the CPAP users in general updated with recalls, manufacturer corrections, and new device and equipment launches.
So we’ll provide updated information in a question and answer format, doing our best to address the most updated manufacturer news, as of November 1, 2022.
What is the status of the Philips Respironics Recall?
In addition to creating tools and resources that can offer support throughout this process, Philips Respironics has also been working to produce repair kits and replacement devices and shipping those out to those affected by the June 2021 recall. Their numbers since October 25, 2022 are as follows:
- 3,950,000 repair kits and replacement devices produced to date globally
- 2,000,000 devices shipped in the US
Their Goal: “We aim to complete the repair and replacement program for the majority of registered patients by December 2022. While we are making progress, we realize that for patients waiting for a repaired or replaced device, progress can’t come fast enough.”
– Philips Respironics
If you are still waiting for a remediated or replacement device, please be patient as Philips continues to work through the process. It is also very likely that this recall will stretch into 2023. If you have not registered your device with Philips, please do so immediately. To register your device, hit the button below:
You can also contact Philips regarding your device status and registration at 1-877-907-7508. If you are unsure which devices were affected by the recall, please see image below:
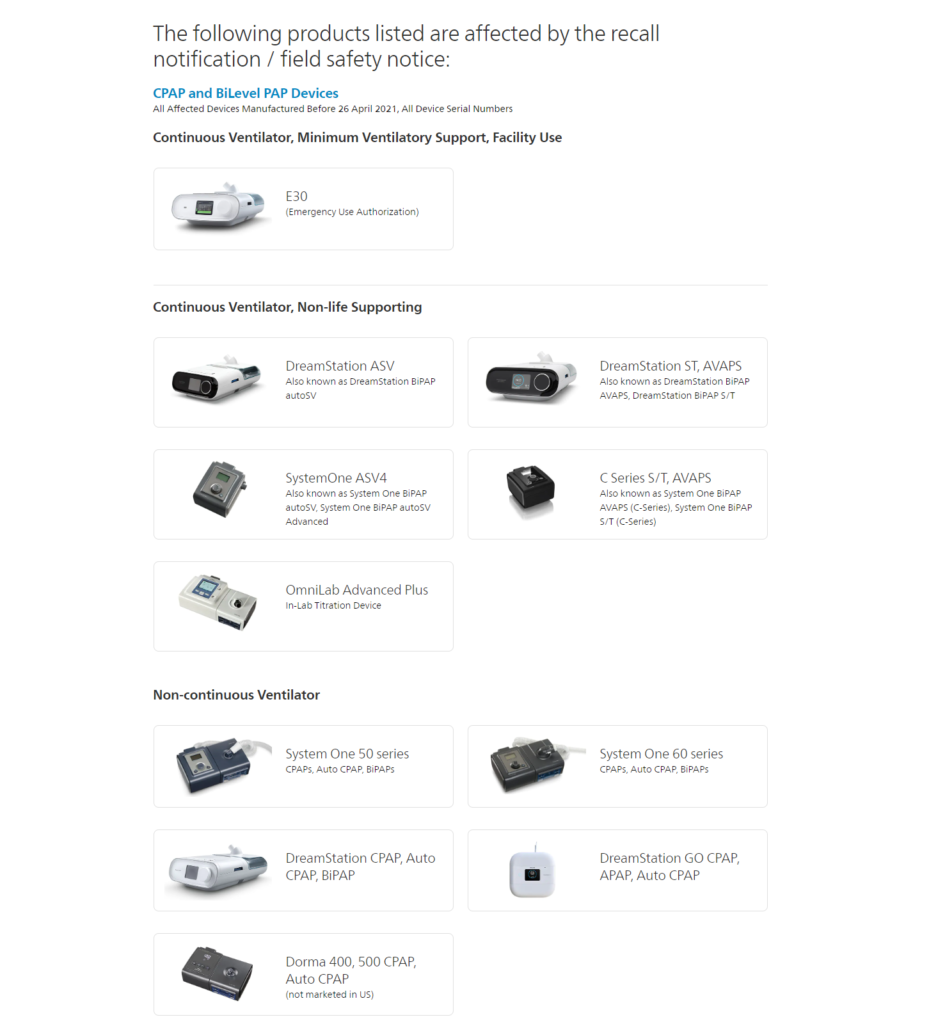
Alternatively, if you live in Idaho, and would like to purchase a non-recalled CPAP Device, please contact our office at 208-323-CPAP (2727).
What are the current recommendations for cleaning your CPAP equipment?

According to Philips Respironics, they are strongly urging their patients NOT to use Ozone and UV Light cleaning products. This recommendation follows the current FDA guidelines, as they have not authorized any products using ozone gas or ultraviolet (UV) light to clean, disinfect, or sanitize continuous positive airway pressure (CPAP) devices and accessories (for example: hoses, masks, tubing and headgear).
“Ozone and UV light cleaning products are not currently approved cleaning methods for sleep apnea devices or masks and should not be used.”
The potential risks of using Ozone and UV Light cleaners:
- The Ozone and UV light cleaning systems can contribute to foam degradation, which is the reason behind the June 2021 recall of millions of sleep apnea and ventilator machines. The foam in the CPA machine was used to quiet the device, but it was discovered that users can inhale or ingest the particles once the foam breaks down, possibly exposing them to toxic chemicals. Some studies have recently shown that occurrences of break down are very low, still any risk regardless of how low should be taken seriously and with an urgent response.
- Ozone cleaners pose an additional risk. Ozone cleaners are designed to keep the ozone gas inside the machine and hoses, but leaks can occur with the various connectors and filters of the CPAP accessories. When leaks occur, ozone gas in the nearby space may temporarily rise to unsafe levels, especially if the space is not well ventilated. According to the FDA, these unintentional and often undetected leaks could lead to nasal, lung or any other type of irritation to the user’s breathing passages. Exposure to high levels of ozone gas also may worsen chronic respiratory diseases, such as asthma, or increase vulnerability to respiratory infection. The FDA has received reports of all the above complaints from using ozone gas cleaners.
- UV Light cleaners also pose various risks. UV Light cleaners can effectively disinfect surfaces but should be used with great care, to ensure the UV light does not escape the surroundings. If not properly shielded, the UV light cleaners do pose a potential health hazard depending on the wavelength, intensity, and exposure time. The risk of unintentional or excessive exposure to UV light during cleaning may put a user at risk of eye injury, skin burns or even an increased risk of skin cancer, according to the FDA. And the UV Light may not be as effective when it comes to disinfecting CPAP devices and equipment as it may be unable to penetrate all areas of the CPAP accessories such as the hoses, masks and connectors.
- For more information, please review:
What are current recommended cleaning instructions for your CPAP Device?

The only routine care a PAP machine requires is regular cleaning or replacement of the air intake filters. This is important to keep the internal parts from accumulating particulates. Disposable filters should be checked at least every other week and generally replaced every 2-4 weeks or when they appear dirty. The non-disposable filter may be rinsed with clear running water if they appear dirty and reinserted into the machine after ample time to air dry. Non-disposable filters should be replaced every six months.
Keep the area around your machine clean. Remove any dust from around the machine to improve the air quality delivered to your machine. Keep the air intake of the machine unblocked. Curtains, bedding, and papers can easily block the air intake of your machine, reducing the airflow and increasing work on the blower motor.
For more information on cleaning your mask and a schedule for replacing CPAP supplies, please visit: https://www.everythingcpap.com/cpap-maintenance-cleaning-faqs/
How do I prepare and mitigate for this and future recalls of my CPAP device, mask and accessories?

Your manufacturer, your physician, your DME supplier are all committed to providing up-to-date safety information about recalls, recommendations and regular updates via email, social media, their websites, and press releases on various media channels. To ensure you personally receive all the pertinent information and remediation instructions, it is best to do the following:
- Register your CPAP device with the manufacturer, which enables the company to provide important updates directly to you, the user, via email, mail or phone/fax. If your contact information changes, login to your user account to notify them of those changes.
- Register your device with your DME supplier and verify that your DME supplier has your most current contact information. If you have received your device from a DME supplier, the manufacturer has that specific device matched to the DME company, so its imperative to keep the lines of communication open with your DME supplier. When an update or change occurs, the DME supplier can reach out to you and provide instructions, a remediated or replacement device to their patients. Everything CPAP personally reached out to each and every one of their patients who was using one of the affected devices of the June 2021 recall.
- The DME supplier also registers each device with the manufacturer and efficiently tracks each device with the corresponding patient’s device by the serial number, make, model and issue date. This database enables our staff to accurately track and contact the affected users.
*This three-fold approach to registration has been implemented to prevent loopholes and a breakdown of communication of manufacturer to the DME Supplier and to the CPAP user.
In the past months, our patients have received brand new Philips Respironics DreamStation 2 Advanced Auto CPAP Machines, as well as the first edition of the DreamStation CPAP & Bi-level Therapy Systems that have been rebuilt and refitted to address the foam degradation issue. If you are currently waiting on your remediated device and still have questions, feel free to contact us.
What are the current recommendations when it comes to the Philips CPAP Masks with magnets?
First off, this voluntary notification by Philips is not a mandatory Class 2 or 3 Recall but a Class 1 Recall, which means the manufacturer notifies its users of a situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death. This means the warning label does not apply to everyone but those with specific conditions, or contraindication.
“A contraindication is a condition under which the device should not be used because the risk of use clearly outweighs any possible benefit. A warning alerts the patients about a situation which, if not avoided, could result in death or serious injury. Additionally, a warning may also describe potential serious adverse reactions and safety hazards. In short, a contraindication tells the user when a device should not be used, and a warning tells the user how to avoid sources of harm in the use of the device.”
– Philips Respironics
Philip Respironics’ voluntary notification refers to the following:
MASK TYPES INCLUDED: Philip Respironics’ voluntary notification refers CPAP Mask with magnetic components. The masks feature magnets to connect components and hold devices in place. Magnets with a magnetic field strength of less than 400 mT are used in the mask, thus its recommended to keep the mask at least 6 inches (approx. 15.24 cm) away from any other medical implants or medical devices that can be impacted by the magnetic fields.
WHO THIS APPLIES TO: Their warnings have been strengthened for patients and their household members, caregivers, and bed partners that may be in close vicinity to patients using the masks.
WHAT CONTRAINDICATIONS are included in the warning:
- Pacemakers
- Implantable cardioverter defibrillators (ICD)
- Neurostimulators
- Magnetic metallic implants/electrodes/valves placed in upper limbs, torso, or higher (i.e. neck and head)
- CSF (cerebral spinal fluid) shunts (e.g., VP (ventriculo peritoneal) shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Metallic splinters in the eye
- Ocular implants (e.g., glaucoma implants, retinal implants)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Metallic gastrointestinal clips
- Metallic stents (e.g., aneurysm, coronary, tracheobronchial, biliary)
- Implantable ports and pumps (e.g., insulin pumps)
- Hypoglossal Nerve Stimulators
- Devices labeled as MR (magnetic resonance) unsafe
- Magnetic metallic implants not labeled for MR or not evaluated for safety in a magnetic field
What are the potential risks? The use of a mask with magnets can potentially impact the functionality or effectiveness of implanted metallic medical devices/metallic objects. Philips reports that out of 17 million distributed masks with magnets, they have only been 16 reports of impacted medical devices including “pacemaker interference or failure, resetting of automatic implantable cardioverter defibrillator, seizures, defibrillator shutting off periodically, arrhythmia, irregular blood pressure, change in heartbeats, and cognitive issues. Philips Respironics has not received any reports of patient death in connection with this matter.” For more information: https://www.usa.philips.com/healthcare/e/sleep/communications/magnet-notification
Should I stop using a mask with magnets? CPAP masks with magnets are still in production and still on the market, as they are still safe to use EXCEPT for those with the above listed contraindications. At this time, only Philips Respironics have released this voluntary notification, but if you are using ANY CPAP mask with a magnet and you are unsure if your situation applies to the contraindications, please speak to your physician or sleep professional without delay. After which, you can work with your DME supplier to provide an alternative mask without magnets.
Stay updated: For continued updates on recalls, notifications and updates, please visit our page: